Electrostatic flocking is the process of directing pre-treated short fibers on a surface of a substrate coated with a binder in a high-voltage electrostatic field, followed by bake-out to integrate the fluff and the substrate cloth. Flocking adhesives are one of the key materials for flocking production, and polyacrylate adhesives are very important in emulsion adhesives and are widely used in coatings, textiles, leather, construction seals, etc. [1-3] ]. In general, the polymerization of acrylic ester-based adhesives requires the dispersion of the polymer in water and other media to form a stable emulsion in the presence of an emulsifier. There is often a certain amount of free emulsifier[4] remaining in the product obtained by polymerization of this method, thereby reducing the adhesion of the emulsion binder on the surface of the substrate, and also giving the surface properties of the emulsion binder, the compactness of the emulsion film, Anti-friction properties and water resistance have caused some negative effects [5]. For the above reasons, soap-free emulsion polymerization methods have been studied in recent years. Cowshed Fan,Cowhouse Exhaust Fan,Poultry House Centrifugal Fans,Dc Fan Co.,LTD , http://www.nsexhaustfan.com
In this paper, a polymerizable emulsifier, maleic anhydride fatty alcohol polyoxyethylene ether monoesters, was used to prepare soap-free acrylate adhesive emulsions and used in electrostatic flocking to study its application properties.
1 Test section
1·1 Materials and Drugs
Maleic anhydride fatty alcohol polyoxyethylene ether monoester (homemade); butyl acrylate (BA) (analytical pure), Changzhou Hongchuang Polymer Technology Co., Ltd.; MMA (Analytical Pure), Shanghai Yujie Trading Co., Ltd. product; Styrene (St) (Analytical Pure), Zhejiang Huatian Fine Chemical Co., Ltd. product; Potassium Persulfate (KPS) (Chemical Pure), Yixing Second Chemical Reagent Factory; Sodium Bicarbonate (Chemical pure), Jinan Huifengda Chemical Co., Ltd. products; Ammonia (industrial grade), Hangzhou Kemei Chemical Co., Ltd. products; Deionized water (homemade); Flocking fabric, Jiaxing Detai Flocking Co., Ltd. products; Nylon fluff (1·1 dtex×1·2 mm), Jiaxing Detai Flocking Co., Ltd.
1·2 Instruments and Equipment
CS501 super constant temperature water bath, Shanghai Sunshine Experimental Instrument Co., Ltd.; JJ-1 type close force electric mixer, Jiangsu Jintan Universal Science Instrument Factory; BZY-Z automatic surface tension meter, Shanghai Hengping Instrument Factory; LB-550 Dynamic Light Scattering Nanoparticle Size Analyzer, Japan Horiba Corporation; JSM-100X Transmission Electron Microscope, Japan JEOL Corporation; Nicolet-5700 Fourier Infrared Spectrometer, ThermoNicolet USA; PyrisDiamond Differential Scanning Calorimeter, USA Perkin Elmer Corporation; flocking prototype, Jinan Xinfeng Flocking Equipment Co., Ltd.; Martin Dyerpin Grinding Instrument, Wenzhou Darong Textile Standard Instrument Factory; AG-1 universal material testing machine, Shimadzu Corporation, Japan.
1·3 Test Methods
1.3.1 Polymerizable Emulsifier Synthesis Process
To a four-necked flask equipped with a thermometer, a stir bar, and a condenser, a predetermined amount of a fatty alcohol polyoxyethylene ether was added, the water was distilled off under reduced pressure, and the temperature was raised to the reaction temperature under a nitrogen atmosphere. Horses were added according to the formulated amounts. The maleic anhydride and the catalyst are reacted for a certain time and neutralized with an aqueous solution of sodium hydroxide to obtain a maleic anhydride fatty alcohol polyoxyethylene ether monoester product.
1.3.2 emulsion polymerization process
Using pre-emulsified seed emulsion polymerization method: Put some polymerizable emulsifier and deionized water into the emulsifier and stir and dissolve at room temperature; homogeneously drop the MMA, BA and St monomers with the mass ratio of 6:6:1 into the above aqueous solution. The mixture was stirred for 30 min to obtain a pre-emulsion; the remaining polymerizable emulsifier, a portion of the initiator aqueous solution, the remaining deionized water, the pH regulator, and 10% pre-emulsion were added to the reactor, heated and stirred, and the temperature was increased. To 75 °C; until the emulsion appears blue, after the temperature dropped, the remaining pre-emulsion and the initiator solution was added dropwise within 2 h and continue to maintain 1 h, until the temperature dropped to below 50 °C, adjust the pH of the emulsion with ammonia Value to neutral.
1·3·3 Flocking process
Slowly add the thickener HIT to the adhesive emulsion under stirring, adjust the viscosity to the desired viscosity, apply it to the flocking base cloth, flock with nylon fluff, and bake at 80°C for 5 minutes before baking at high temperature bake.
1·4 Test Methods
1.4·1 Conversion rate of monomer
In the course of the reaction, a certain amount of emulsion is weighed into a dry clean weighing bottle, a drop of a 5% hydroquinone ethanol solution is added, and placed in an oven at 115° C. for drying for 4 hours, taken out and dried. The device was cooled to room temperature and weighed. The conversion rate of the monomer is calculated as follows: 
1·4·2 Particle Size of Emulsion Particles
A small amount of emulsion was taken in a test bottle, diluted with distilled water, and tested for particle size using a Horiba LB-550 dynamic light scattering nanoparticle sizer.
1·4·3 gel rate of emulsion
After the completion of the polymerization reaction, the emulsion was filtered through a 200 mesh nylon mesh screen, and the filtered residue, gel material on the reactor and stirrer bar were collected, dried in a 115°C oven to constant weight, and weighed. The gel rate is calculated as follows: 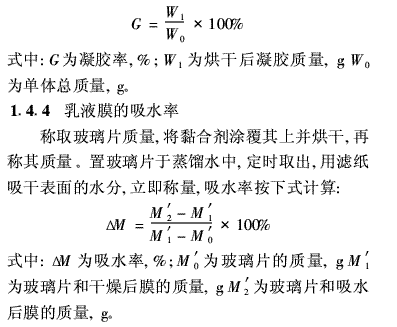
1·4·5 Surface tension of emulsion
The emulsion was diluted to a certain concentration and the surface tension was measured by a BZY-Z automatic surface tension meter at 20°C.
1·4·6 Electrolyte Stability of Emulsion
Add 8 mL of polymer emulsion sample into a 10 mL graduated test tube, and add 2 mL of CaCl2 solution at concentrations of 0·05, 0·10, 0·50, and 1·00 mol/L, respectively. Shake well and stand. 48 h, observed changes in the emulsion.
Infrared Spectroscopy (FT-IR) Analysis of 1.4.7 Emulsion Films
The emulsion was coated on slides, dried in a 60°C drying oven, and its film was taken. The functional groups of the emulsions were characterized by a Fourier infrared spectrometer (Nicole 5700) and the measurement range was 4 000 to 500 cm-1.
Transmission Electron Microscopy (TEM) Analysis of 1·4·8 Emulsion Particles
The sample to be tested was diluted to a certain degree, and a few drops on a copper mesh were observed with a JSM-100X transmission electron microscope to observe the particle morphology and photographed.
DSC Analysis of 1·4·9 Emulsion Films
The copolymer film was accurately weighed and subjected to differential thermal analysis under a nitrogen atmosphere using a PyrisDiamond differential scanning calorimeter from Perkin Elmer, USA, at a heating rate of 10°C/min.
1·4·10 Flocking Fastness Test of Flocked Fabrics
The flocking fastness of the flocked fabrics was tested with a Martindale sander according to the FZ/T 64011-2001 standard for electrostatic flocking fabrics. The greater the number of times the flocked fabric was rubbed (even if fluff did not fall off), the better the flocking fastness.
1·4·11 Breaking strength of flocking fabrics
In accordance with GB/T 3923·1-1997 "Measurement method for tensile strength breaking strength and elongation at break of textile fabrics (strip method)", it was tested on an AG-1 type universal material testing machine.
2 Results and Discussion
Synthesis and Characterization of 2.1 Non-soap Adhesive Emulsion
Application of 2.1·1 Series Polymerizable Emulsifier in Emulsion Synthesis
In the emulsion polymerization system, the polymerizable emulsifier functions to emulsify the oil phase monomer to form micelle particles having a solubilizing effect, stabilize the emulsion particles during the polymerization reaction, and prevent gelation during emulsion polymerization. The polymerizable emulsifiers have different hydrophilic-lipophilic balance values ​​(HLB values), and the emulsification of the oil phase monomer and the stabilizing effect of the emulsion particles are also different. A series of maleic anhydride fatty alcohol polyoxyethylenes with different HLB values ​​were studied at a monomer mass fraction of 20% (percent emulsion composition), initiator usage of 1% (on monomer weight) and a polymerization temperature of 75°C. The effects of ether monoesters on the properties of the emulsions are shown in Table 1. 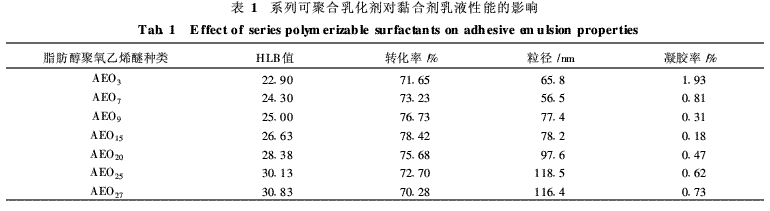
It can be seen from Table 1 that with the increase of oxyethylene chains in maleic anhydride fatty alcohol polyoxyethylene ether monoesters, the HLB value gradually increases, the conversion rate of monomer gradually increases, and the gelation rate decreases; After the number of oxyethylene chains reaches 15, the conversion rate of monomer gradually decreases, the gelation rate gradually increases, and the emulsion performance deteriorates. This is because the HLB value of the polymerizable emulsion affects the reaction rate of the emulsion polymerization and the stability of the system. When the HLB value of the polymerizable emulsifier is low, the emulsion particles easily agglomerate in the emulsion polymerization process and even cause emulsion demulsification; when the HLB value of the polymerizable emulsifier is high, the chain growth rate during the emulsion polymerization is slow, and the emulsion polymerization converts. The rate is low [6]. It can be seen that the polymerizable emulsifiers of AEO15 esterified with maleic anhydride are suitable for emulsion synthesis.
The effect of the amount of polymerizable emulsifier 2.1 on the properties of emulsion
As the main body of a stable emulsion system, the amount of polymerizable emulsifier (on the weight of monomers) has an important influence on the properties of the binder emulsion. The effect of the amount of polymerizable emulsifier on the properties of the adhesive emulsion was investigated under the conditions of a monomer mass fraction of 20%, an initiator dosage of 1%, and a polymerization temperature of 75°C. The results are shown in Table 2. 
It can be seen from Table 2 that when the amount of the polymerizable emulsifier is less than 8%, the conversion rate of the monomer in the system is relatively low, and the particle size of the emulsion particles is large; when the amount thereof reaches 8%, the conversion rate of the monomer increases. The particle size of the emulsion particles becomes smaller. This is because when the amount of the polymerizable emulsifier is small, the formation of a large amount of emulsion particles during the nucleation stage is limited, the conversion rate of the polymerization monomer is low, and the particle size of the emulsion particles is large. In addition, due to insufficient emulsification, the polymerizable emulsifier cannot completely cover the entire emulsion particles to maintain its stability, and the surface charge density is low, the hydration layer is thin, and the particles easily collide and coagulate. When the polymerizable emulsifier is used in an amount of 8% to 10%, a large amount of emulsion particles can be formed at the initial stage of the polymerization to accelerate the polymerization reaction rate, and the polymerizable emulsifier can tightly coat the surface of the emulsion particles to improve the stability thereof. The conversion rate and the particle size of the emulsion particles are also small. Therefore, the amount of the polymerizable emulsifier is 8%.
Effect of 2.1·3 Monomer Mass Fraction on Performance of Adhesive Emulsion
When the monomer mass fraction in the emulsion polymerization system is large, due to the presence of a hydration double layer on the surface of the particles, the movement resistance between the particles is increased and the viscosity is increased, thereby affecting the stability of the emulsion. Therefore, the amount of the polymerizable emulsifier can be used. For 8%, initiator dosage of 1% and polymerization temperature of 75°C, the effects of different monomer mass fractions on the properties of the binder emulsion were investigated. Table 3 shows the results. 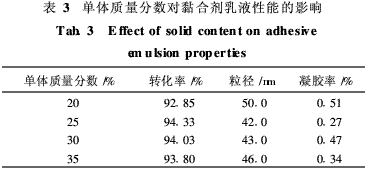
From Table 3, it can be seen that as the monomer mass fraction increases, the gel fraction of the polymer product becomes smaller, the particle size of the emulsion particles is 40 to 50 nm, the emulsion stability is good, and the monomer conversion rate does not change significantly. This is because the number of emulsion particles in the nucleation stage hardly changes with the increase of the monomer mass fraction, but the volume of the emulsion particles will increase with the increase of monomer mass fraction, and will increase with the monomer mass fraction. There is also the viscosity of the system. The increase in viscosity affects the relative motion between the emulsion particles and is not conducive to the stability of the polymerization system. In addition, an increase in the mass fraction of monomer will also increase the time for monomer droplets to diffuse from the aqueous phase into the emulsion particles and polymerize them, resulting in a lower conversion rate of the monomer and a longer reaction time [7]. Therefore, choose a monomer mass fraction of 25%.
Analysis of Water Absorption of 2·1·4 Soap-free Emulsion Film
Adhesive emulsion sample B6 prepared from adhesive emulsion samples B1 to B5 prepared from different amounts of maleic anhydride fatty alcohol polyoxyethylene ether (15) monoesterified esters and emulsifiers TX-30 and SDS was allowed to stand at room temperature. The film was formed, immersed in deionized water and taken out after a certain period of time. The water absorption of the film was weighed and plotted. The results are shown in FIG. 1 . As can be seen from Figure 1 (a), with the increase of the amount of polymerizable emulsifier 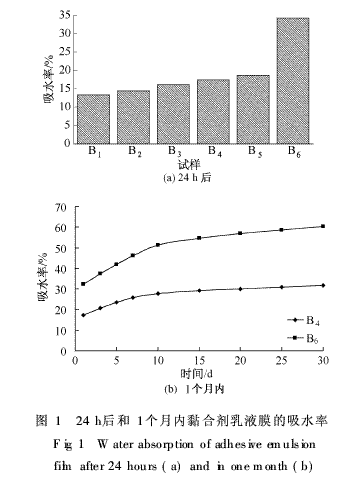 The water absorption after the emulsion film (samples B1~B5) 24 of the synthetic soap-free adhesive gradually increased to 18.64%, and the emulsion of the adhesive prepared by the combination of the emulsifier TX-30 and SDS The water absorption of the membrane was 34.23%.
The water absorption after the emulsion film (samples B1~B5) 24 of the synthetic soap-free adhesive gradually increased to 18.64%, and the emulsion of the adhesive prepared by the combination of the emulsifier TX-30 and SDS The water absorption of the membrane was 34.23%.
From Fig. 1(b), the change trend of the water absorption rate of the emulsion film within one month shows that the sample B4 (the polymerizable emulsifier dosage is 8%) has a slow water absorption rate, and the water absorption rate only increases by half of the initial value within 30 days; The water absorption rate of Sample B6 was fast, and it was always in an increasing trend, and the final water absorption rate increased nearly 1 times. It can be seen that replacing the conventional small molecule emulsifier with a polymerizable emulsifier can significantly improve the water resistance of the emulsion film. This is because the polymerizable emulsifier can be chemically bonded to other reactive monomers on the surface of the polymer without migration, and the surface of the emulsion formed only contains a small amount of hydrophilic groups, thereby reducing the water absorption of the emulsion film. , weaken the permeability of water molecules to the membrane and improve the water resistance of the polymer emulsion membrane [8]. On the contrary, the surface of emulsion membranes prepared from traditional emulsifiers is enriched with a large number of emulsifier molecules, and the hydrophilic groups of these emulsifiers can adsorb large amounts of water molecules; in addition, traditional emulsifiers can migrate from the emulsion membrane to the aqueous phase. Numerous pores formed on the emulsion membrane facilitate the penetration of water molecules to further reduce the water resistance of the emulsion membrane.
Analysis of Electrolyte Stability of 2.1·5 Soap-free Adhesive Emulsion
Adhesive emulsion samples B1 to B5 prepared using different amounts of maleic anhydride fatty alcohol polyoxyethylene ether (15) monoesterified esters and binder emulsions B6 produced by using emulsifiers TX-30 and SDS for different concentrations The test results of CaCl2 stability performance are shown in Table 4. 
The results in Table 4 show that when the concentration of CaCl2 is 0.5 mol/L, a large amount of precipitates and even gels are observed in the emulsion samples B1 and B2 prepared from polymerizable emulsifiers; the composite emulsifiers are prepared using TX-30 and SDS. The emulsion is less stable to the electrolyte, gel appears, and the emulsion is completely destroyed. It can be seen that the emulsion anti-electrolyte properties obtained by the polymerizable emulsifier are superior to the conventional emulsifiers. This may be because: When the electrolyte concentration increases, the probability that the anti-particles will diffuse to the surface of the emulsion particle increases, and the concentration of the hetero-ion in the adsorption layer will increase, resulting in a decrease in the potential of the double-layer and a decrease in the stability [9] . In a soap-free emulsion polymerization system, a polymerizable emulsifier is copolymerized and bound to the surface of the emulsion particles, and the molecule contains a certain amount of an oxyethylene hydrophilic group that is stable to the electrolyte, and the more polymerizable emulsifiers on the surface of the emulsion particles, The electrolyte stability of the emulsion is also better.
TEM Analysis of 2·1·6 Soap-free Adhesive Emulsion Particles
The emulsion particles of the binder were magnified 100,000 times under transmission electron microscopy (TEM). The morphological structure is shown in Fig. 2, and the particle size distribution of the particles is shown in Fig. 3. 
From figures 2 and 3, it can be seen that the emulsion particles are fine and regular 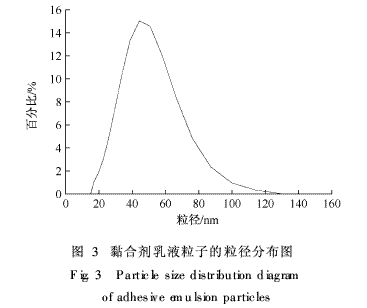
It is spherical and has a uniform distribution with an average particle size of about 50 nm. The TEM image also shows that the boundary of the emulsion particles is ambiguous, and it can be inferred that the polymerizable emulsifier is mainly bonded to the surface of the emulsion particles, and the polyoxyethylene segment in the molecules has also been dissolved in the water phase.
2.1.7 DSC analysis of soap-free adhesive emulsion films DSC analysis was performed on the soap-free adhesive emulsion films. The results are shown in FIG. 4 . As can be seen, there is only 1 glass transition on the DSC chart 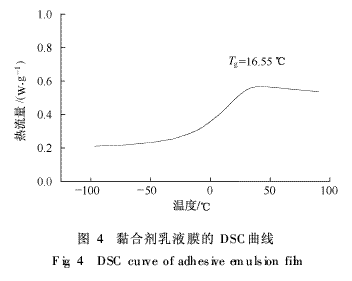
In the variable interval, the Tg is 16.55°C. It is known that the Tg of the homopolymers PBA, PMMA, and PSt are -57, 105, and 100°C, respectively, and there is no such transition in the DSC curve, indicating that each reaction monomer and the horse The anhydride anhydride fatty alcohol polyoxyethylene ether monoester compound copolymerizes well and there is no corresponding homopolymer.
FT-IR Analysis of 2·1·8 Soap-Free Emulsion Membrane
The FT-IR spectrum of the synthetic soap-free adhesive emulsion film is shown in Figure 5. As can be seen from the figure, the C—H stretching vibration peak on the benzene ring is at 3 029-9 cm −1; the asymmetric and symmetric stretching vibration peak of —CH 3 is at 952·3 and 2 873·4 cm −1; · 0 cm-1 is -CH2 
Asymmetric stretching vibration peak; The absorption peak at 1 725·0 cm-1 is the stretching vibration peak of the carbonyl CO in the ester group; 1 602·1, 1 493·7, 1 451·7 cm-1 are in the benzene ring CC The absorbance peaks are at 1383.0 cm-1 at the end-CH3; 1143-4 and 1 028-9 cm-1 are the absorption peaks at C-O-C; 991.5 and 842.4 cm. -1 is -CH3 and -CH2 out-of-plane bending vibration absorption peaks; 960·7, 941 ·1 cm-1 is a characteristic peak of butyl acrylate; 758·4, 700 · 5 cm-1 is mono-substituted benzene C-H out-of-plane vibration absorption peak. This shows that copolymerization has occurred between the respective monomers and the maleic anhydride fatty alcohol polyoxyethylene ether monoester compounds.
Analysis of the Surface Tension of 2.1·9 Soap-free Adhesive Emulsion
In a soap-free adhesive emulsion polymerization system, a polymerizable emulsifier is copolymerized with a main reaction monomer. If the polymerizable emulsifier has been completely copolymerized to the polymer, the polymer emulsion has no free polymerizable emulsifier in the aqueous phase, and the surface tension of the aqueous solution of the polymer emulsion should be close to that of water. Therefore, the degree of polymerization of the polymerizable emulsifier can be qualitatively judged by measuring the surface tension [10-11] of the aqueous emulsion solution. The surface tension test results of the binder emulsions prepared from polymerizable emulsifiers are shown in Table 5.
It can be seen from Table 5 that the surface tension of the adhesive emulsion prepared from the polymerizable emulsifier is similar to that of pure water (66.67-68.34 mN/m), but the concentration of the polymerizable emulsion solution is higher than that of the pure water. 
The surface tension (40·54 mN/m) above the CMC value is much higher, indicating that only a very low concentration of polymerizable emulsifier is present in the aqueous phase. The emulsions prepared from traditional emulsifiers SDS and TX-30 had surface tensions (38·82 mN/m) that were very close to the surface tension of the aqueous solution (33·46 mN/m), indicating that the SDS and TX-30 were physically Adsorption on the surface of emulsion particles.
The application of 2·2 soap-free adhesive emulsion in electrostatic flocking
Using 0·11 tex×1·2 mm nylon fleece and flocking base cloth, a soap-free adhesive emulsion made of a polymerizable emulsifier is used for electrostatic flocking of flocking adhesives, focusing on the amount of adhesive applied and baking. The influence of process factors, such as baking temperature and time, on the application properties of the adhesives, in order to determine the best process for electrostatic flocking.
Effect of 2·2.1 Application of Adhesive on the Properties of Flocking Fabrics
The main function of the adhesive is to connect the fluff and the backing cloth. The amount of adhesive applied directly affects the flocking fastness, breaking strength, air permeability and handfeel of the flocked fabric. The effects of the amount of binder applied on the flocking fastness and the breaking strength of the flocked fabrics were studied under the conditions of a baking temperature of 155°C and a baking time of 5 min. Table 6 shows the results. 
It can be seen from Table 6 that the flocking fabric has improved flocking fastness and breaking strength as the amount of adhesive applied increases. As the adhesive layer of the flocked fabric, the amount of adhesive applied determines the flocking and breaking strength of the fabric. If the amount of adhesive applied is small, the adhesive layer of the fabric is thin, the villi are implanted lightly, and the villi are easy to pull out under the action of external force; if the applied amount is too high, the adhesive layer becomes thick and the villi are implanted too deeply, and when the friction is very easy, Break and affect the fastness. In addition, an excessive amount of adhesive can also affect the feel of the fabric, so the amount of adhesive applied is 300 to 400 g/m2.
The effect of curing temperature on the properties of flocking fabrics after 2·2·2 baking
The effect of curing temperature on the flocking fastness and breaking strength of the flocked fabric was investigated under the conditions of a sizing amount of 300 g/m2 and a baking time of 5 min. The results are shown in Table 7. 
The curing temperature of the adhesive is the main factor that determines the performance of the adhesive film. When the temperature is low, the film is not completely cured, resulting in low film strength; at high temperatures, the film can be fully cured and the film fastness is enhanced. From Table 7, it can be seen that as the baking temperature is increased, the flocked fabric has increased flocking strength and breaking strength. When the temperature reached 160°C, the flocking fastness of the fabric remained basically unchanged, and the breaking strength also changed little. This indicates that the film has been cured more completely, and increasing the temperature has little effect on its fastness. Therefore, the baking temperature was determined to be 160°C.
The Effect of 2·2·3 Baking Time on the Properties of Flocking Fabrics
The effects of baking time on the flocking fastness and breaking strength of the flocked fabrics were studied under the conditions of 300 g/m2 of binder application temperature and 160°C baking temperature. The results are shown in Table 8. 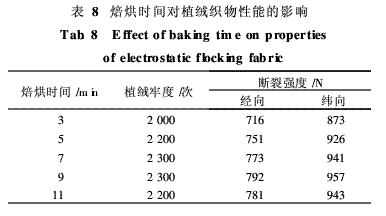
From Table 8, it can be seen that the flocking fastness and breaking strength of the flocked fabrics gradually increase with the extension of baking time, but the fastness and strength of the fabrics no longer increase after baking for 7 min, and the temperature is too high for a long time. The role of easy to dry hair, destroy the feel, and even yellowing. Therefore, under the premise of ensuring the fastness, the baking time was determined to be 7 min.
Therefore, the best process for determining flocking is: application of adhesive 300 g/m2, baking temperature 160°C, baking time 7 min. The soap-free adhesive emulsion synthesized by the polymerizable emulsifier was flocked with the best flocking process and tested for the quality index of the flocked fabric. The flocking fastness was 2 300 times and the breaking strength was high. According to the FZ/T 64011-2001 "Electrostatic Flocking Fabrics" standard (generally, flocking fabrics for clothing require more than 2,000 flocking fastnesses, and flocking fabrics for decorative use require more than 6,000 flocking fastnesses), the flocking The flocking fastness of the fabric has reached the requirements of clothing flocking fabrics.
3 Conclusion
1) A pre-emulsified seed polymerization method was used to emulsify maleic anhydride and a series of fatty alcohol polyoxyethylene ether monoesters to prepare emulsions of methyl methacrylate, butyl acrylate, and styrene copolymerized soap-free adhesives. The related test results show that the synthesized soap-free adhesive emulsion particles are regular in shape and uniform in size distribution; the electrolyte stability of the emulsion and the water resistance of the emulsion film are greatly improved compared to those produced by traditional emulsifiers; the polymerizable emulsifiers can be greatly improved; Copolymerization with the monomer is well.
2) Using a flocking process with a binder application amount of 300 g/m2, a baking temperature of 160[deg.] C., and a baking time of 7 minutes, a polymerizable emulsifier-based adhesive is used for electrostatic flocking and testing for flocking The flocking fastness and breaking strength of the fabric indicate that the flocking fastness of the binder meets the requirements of flocking fabrics for clothing.
Synthesis of soap-free adhesive emulsion and its application in electrostatic flocking